Graphene battery self-charging using environmental heat
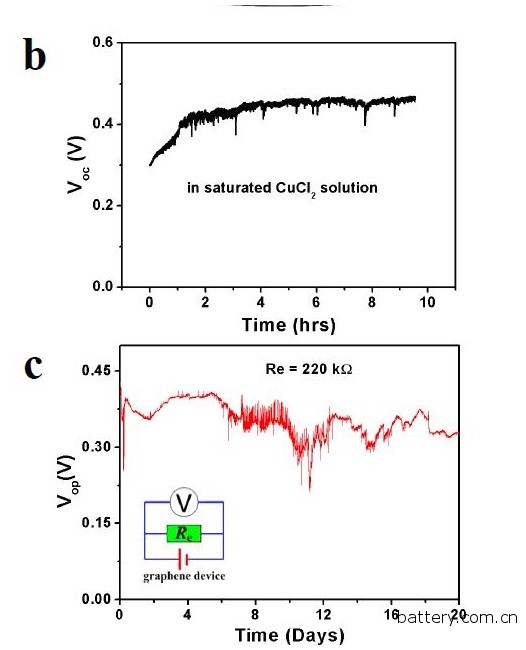
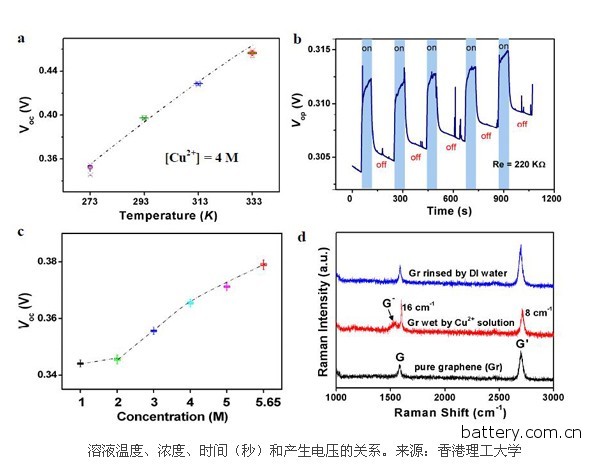
Graphene battery self-charging using environmental heat This is an interesting idea for making batteries. The thermal motion of ions in aqueous solutions is enormous, reaching hundreds of meters per second at room temperature. But few people have studied this process, and no one has studied how it might produce electricity. Zihan Xu, who conducted this research, was from Hong Kong Polytechnic University. He and several of his companions not only studied the process, but also seemed to have mastered it. The relationship between time (hours, days) and voltage generation of a graphene battery in a saturated copper chloride solution. Source: Hong Kong Polytechnic University These guys have been made into circuits that contain LEDs that are wired to ribbon graphene. They simply placed graphene in a copper chloride solution for observation. Sure enough, the LED lights up. In fact, they need six graphene circuits to form a series, so that the required 2V can be generated, and the LED light can be illuminated to get the picture. Xu Zihan and colleagues said that this is the case here. Copper ions have a double positive charge and the rate of passage through the solution is about 300 meters per second because of the thermal energy of the solution at room temperature. When an ion violently strikes a graphene ribbon, the collision produces enough energy to cause the electrons that are not in the field to leave the graphene. These electrons have two choices: they can leave the graphene band, combine with copper ions, or pass through graphene to enter the circuit. It turns out that flowing electrons are faster in graphene than they pass through the solution, so the electrons naturally choose the path through the circuit. It is this that illuminates the LED lights "the electrons that are released tend to pass through the graphene surface rather than entering the electrolyte. Our equipment produces voltage like this," Xu Zihan said. Therefore, the energy generated by this device comes from the heat of the surrounding environment. These guys say they can increase the current, just heat the solution, or use ultrasonic waves to accelerate the copper ions. They even claim that their graphene batteries can last up to 20 days, relying on the heat alone. However, there is an important question mark. Another assumption is that a chemical reaction produces electricity, just like a normal battery. However, Xu Zihan and colleagues said that they ruled out this because several sets of control experiments were conducted. However, these are introduced in some supplemental materials that do not seem to be on the arXiv website. They need to disclose this, before they can make a serious statement. From the perspective of surface value, this seems to be a very important outcome. Others also generate overcurrents in graphene, but only let water flow through it, so this is not really surprising, and moving ions can also produce this effect. This heralds a clean green battery that relies solely on ambient heat. Xu Zihan and colleagues said: "This represents a huge breakthrough, the study is self-driven technology." We hope they are correct. But at least for now, people still can't draw conclusions. Excavator Swing Bearing,Excavator Turntable Bearing,Wanda Slewing Bearing Slewing Bearing Co.,Ltd. , http://www.slewingring-bearings.com